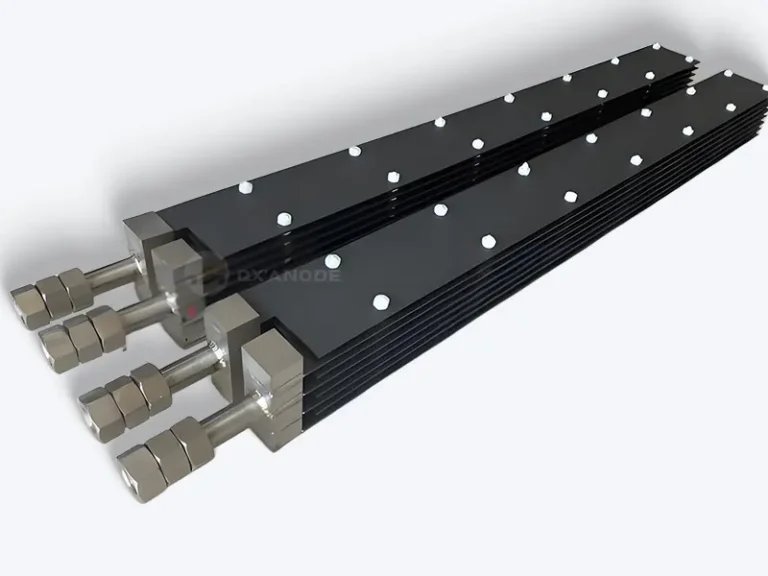
Titanium Anodes: From Material Innovation to Industrial Applications


Basic Characteristics of Titanium Anodes
Titanium anodes are electrochemical electrode materials made from industrially pure titanium and platinum-group precious metals, and they are responsible for electrocatalytic oxidation in the electrochemical industry. The basic characteristics of titanium anodes include excellent electrical conductivity, corrosion resistance, mechanical strength, as well as good processing and welding properties, making them electrode materials in modern electrolysis and electroplating technologies.
Titanium anodes have excellent electrochemical performance, with low chlorine evolution and oxygen evolution potentials, stable cell voltage, and can effectively reduce energy consumption during the electrolysis process.
In the application of electrolytic copper foil, the stability of the cell voltage of titanium anodes can improve the uniformity of the thickness of the produced foil. In terms of corrosion resistance, after the surface of the titanium substrate is coated with precious metal oxides, it can maintain long-term stability in strong acid, strong alkali and chloride ion environments.
The preparation process of titanium anodes includes three major steps: substrate treatment, intermediate layer preparation, and coating sintering.
The substrate needs to undergo sandblasting and acid etching for surface roughening to enhance coating adhesion. The intermediate layer usually uses precious metal oxides such as iridium and ruthenium, which are applied followed by thermal decomposition and high-temperature sintering.
The third-generation metal oxide-coated titanium anode has a high level of technological maturity, and its preparation difficulty lies in the control of coating uniformity and anti-spalling properties. For example, the integrated raw foil machine adopts an embedded process with 1mm titanium electrode plates, but the coating is prone to spalling, which affects the quality of ultra-thin copper foil; the back-pull process is more stable and suitable for the production of ultra-thin copper foil.
Application of Titanium Anodes in Electrochemical Industry
Titanium anodes, with their excellent performance, form the basis for their wide application in the electrochemical industry. Their application scope covers multiple electrochemical industrial fields such as chlor-alkali, electrodeposition of metals, electrolytic copper foil, aluminum foil formation, electrolytic chlorine production, and sewage treatment. Titanium anodes have promoted technological progress in the electrochemical industry and optimized the production efficiency of electrolysis and electroplating by improving current efficiency, reducing energy consumption, and extending service life.
- Chlor-alkali industry: Ruthenium-iridium-titanium anodes are the core materials for the electrolytic production of chlorine gas, sodium hydroxide, and hydrogen gas. They avoid electrolyte contamination, and the product purity is increased by more than 15%.
In the chlor-alkali industry, the working voltage of ruthenium-iridium-titanium anodes is significantly reduced, resulting in an approximate 20% decrease in electrical energy consumption. At the same time, they have strong dimensional stability and can resist the dual corrosion of chlorine and alkali. - Electrodeposition of non-ferrous metals: Iridium-tantalum-titanium anodes are used in the electrolysis of non-ferrous metals such as copper, zinc, and nickel. The stability of the iridium oxide coating extends the anode life to 2-3 times that of traditional lead anodes.
In the field of electrodeposition of non-ferrous metals, tantalum-iridium-titanium anodes exhibit the characteristic of low oxygen overpotential in sulfuric acid solutions. The iridium oxide coating can remain stable for a long time, preventing the dissolution of anode materials. - Sewage treatment and sanitary disinfection: Ruthenium-titanium anodes are widely used in sodium hypochlorite production and sewage treatment, with a current efficiency of over 85% and a 25% reduction in the production cost of disinfectants.
In the field of sewage treatment, ruthenium-iridium-titanium anodes have high current efficiency and low chlorine evolution potential in a chlorine-evolving environment. The electrode substrate can be reused and there is no risk of medium pollution. These characteristics enable titanium anodes to have a much longer service life than traditional materials in highly corrosive environments, reducing maintenance costs by more than 30%.
Comparison between Titanium Anodes and Traditional Anode Materials
The electrical conductivity of titanium anodes is significantly better than that of traditional materials, and their low resistance property can effectively reduce electrical energy loss. Titanium materials have high strength and low density, which can withstand high pressure and fluid impact in the electrolytic cell, preventing electrode deformation or fracture.
Titanium anodes can be custom-processed into different sizes and shapes according to equipment requirements, suitable for applications ranging from small laboratory devices to ten-thousand-ton industrial production lines. Common titanium anodes can be customized into plate, mesh, rod, or tube structures to meet diverse industrial applications.
Environmental characteristics of titanium anodes
- The base material can be reused. Electrodes such as ruthenium-titanium and ruthenium-iridium-titanium can be recycled multiple times after repair when their coatings fail.
- No pollution, avoiding electrolyte contamination caused by the dissolution of traditional electrodes. In the fields of sewage treatment and food disinfection, titanium anodes can ensure that no heavy metals are precipitated during the production of disinfectants such as sodium hypochlorite.
Preparation technology of titanium anodes
Titanium anode substrate mateTitanium, with its characteristics of high strength, corrosion resistance, and low density, has become an ideal matrix material for anode preparation.
Core indicators required for titanium anode substrates
- Electrochemical stability: Titanium materials with Ti ≥ 99.5% are used to eliminate the interference of impurities on electrochemical reactions, ensuring the stability and catalytic activity of the electrode.
- Mechanical strength: The titanium matrix must withstand the fluid pressure and mechanical stress inside the electrolytic cell to prevent electrode deformation.
Surface treatment: increases coating adhesion by more than 25%.
- Sandblasting process: Using quartz sand particles to impact the surface of the substrate, forming a roughness with an Ra value of 1.5-3.0μm.
- Pickling process: Use oxalic acid, sulfuric acid, etc. to remove the surface oxide layer and contaminants, so that the surface energy is increased to more than 30mN/m.
- The substrate treatment parameters must strictly match the coating process.


Table 1:Titanium Anode Preparation Process Flow
Process steps | Key parameters | Technical key points | Application effect |
Selection of Substrate | Purity ≥ 99.5% | Ensure the performance of titanium anodes | Stable electrochemical performance |
Mechanical processing | Dimension control | Ensure geometric accuracy | Optimize current distribution |
Sandblasting treatment | — | Increase the surface roughness | Improve coating adhesion |
Pickling treatment | Oxalic acid/sulfuric acid solution | Remove oxides and contaminants | Clean the surface |
Coating preparation | RuO2-IrO2/IrO2-Ta2O5 | Thermal decomposition oxidation/sintering | Electrocatalytic active layer |
Performance testing | Electrochemical testing | Evaluate catalytic activity | Ensure service life |
Titanium Anode – Production Process Control
The production of titanium anodes must follow a standardized technological process, including steps such as substrate pretreatment, coating preparation, coating application, sintering, and surface treatment.
- Pretreatment of titanium anode substrate: Improve the surface roughness of the titanium substrate and enhance coating adhesion through processes such as sandblasting and pickling.
- Preparation of titanium anode coating: Precisely mix precious metal oxides such as ruthenium and iridium with binders to ensure uniform dispersion of active components.
- Titanium anode coating process: The thermal decomposition oxidation method is adopted, and a dense oxide coating is formed through multiple brushing-sintering cycles.
The quality of titanium anodes needs to be inspected through multiple dimensions to ensure reliability.
Qixin Titanium Industry has established a full-process quality inspection system, monitoring everything from raw material purity to finished product performance to ensure the stable operation of titanium anodes in electrolysis.

Performance of Titanium Anodes
Titanium anodes exhibit excellent catalytic performance in electrochemical processes, with their core advantages reflected in three dimensions: catalytic activity, selectivity, and stability. Catalytic activity directly determines electrolysis efficiency. For example, ruthenium-iridium-titanium anode plates significantly reduce the operating voltage in the chlor-alkali industry, cutting energy consumption by approximately 20%. Moreover, their high current density characteristic enables them to reach 17 A/m² in diaphragm-based chlor-alkali production, which is twice the level of graphite electrodes. This high efficiency stems from the low overpotential property of the noble metal oxide coating, which can effectively reduce the activation energy of chlorine evolution or oxygen evolution reactions.
Titanium anodes achieve efficient and directional catalysis of target reactions through material composition regulation. Ruthenium-titanium anode plates have a catalytic efficiency of over 90% for chlorine gas generation in a chlorine-evolving environment while inhibiting the occurrence of side reactions; tantalum-iridium-titanium anode plates have a reduction selectivity of over 85% for metal ions such as copper and zinc during the electrolysis of non-ferrous metals in sulfuric acid. This precise selectivity benefits from the adsorption regulation of specific reaction intermediates by the coating’s microstructure. For example, iridium oxide coatings preferentially adsorb sulfate ions in an acidic environment, thereby improving the purity of metal electrolysis.
Corrosion resistance and stability
The corrosion resistance of titanium anodes is a key indicator of their ability to work stably for a long time in practical applications. In highly corrosive media such as chlor-alkali electrolytes, sulfuric acid solutions, and sewage treatment environments, the combination of the base material and coating of titanium anodes determines their corrosion resistance. For example, ruthenium-iridium-titanium anode plates perform excellently in the dual corrosive environment of chlorine and alkali, which can effectively prevent the dissolution of the base material. When tantalum-iridium-titanium anode plates are electrolyzed in sulfuric acid solutions, their iridium oxide coatings can remain stable for a long time, significantly extending the service life of the electrodes. Experimental data show that the working voltage of titanium anodes with ruthenium-iridium coatings in the chlor-alkali industry can be reduced by about 20%, which indirectly reflects the reduction in their corrosion loss.
Application fields of titanium anodes
- Chlor-alkali industry: 85% of chlor-alkali enterprises worldwide adopt its electrolyzers, with a single electrolyzer capable of producing 100,000 tons of chlorine gas annually.
- Organic synthesis: In the production of fine chemicals such as ethylene glycol and caprolactam, the catalytic selectivity exceeds 98%, which reduces by-products and lowers costs.
- Water electrolysis for hydrogen production: As the core catalyst carrier of proton exchange membrane electrolyzers, the IrO₂ coating enables hydrogen production efficiency to exceed 75%.
- Energy storage field: in vanadium flow batteries.
- Wastewater treatment: Electrochemical redox can remove heavy metals (removal rate ≥ 90%) and refractory organic pollutants (including phenols, antibiotics, etc., with a removal rate ≥ 90%).
- Electroplating and surface treatment: Used for precision electroplating of gold, silver, chromium, etc. The uniformity of the coating is controlled within ±0.1μm, and the anode sludge rate is reduced by 50%.
- Chlorine production by electrolysis: Efficient and stable core electrolytic components.
- Cathodic Protection: A Long-term Protection Carrier in Extreme Environments.
- PCB copper plating: It is a core material for achieving high-precision hole metallization and surface copper plating. Its excellent catalytic activity and stability can ensure the uniformity and density of the copper plating layer.
- Hydrometallurgy: Titanium anodes are widely used in the electrolytic extraction and refining processes of non-ferrous metals and rare metals such as copper, nickel, cobalt, gold, and silver.