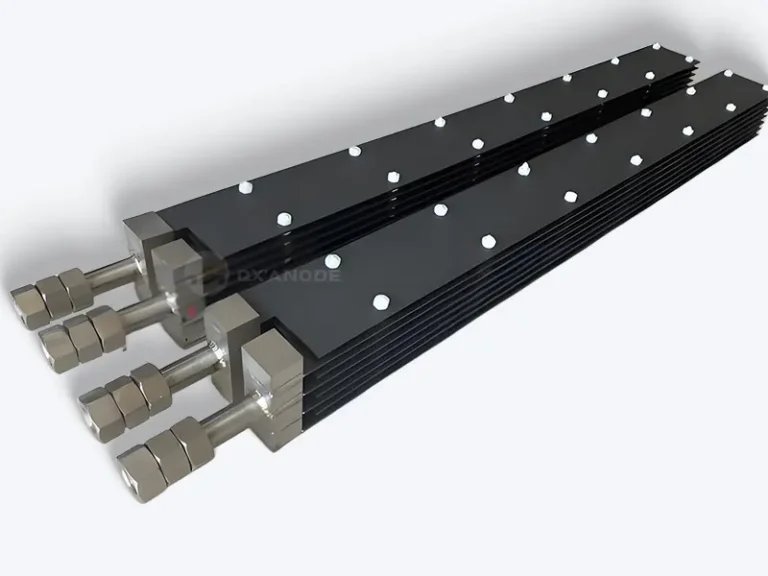
Selection and Usage Precautions of Ruthenium Iridium Anodes
Professional guide to help you fully unleash the excellent performance of ruthenium iridium titanium anodes
Introduction to Ruthenium Iridium Titanium Anode
In the modern field of electrochemistry, ruthenium-iridium-titanium anodes occupy a pivotal position due to their unique properties and are widely used in the electrochemical sector. From the chlor-alkali industry to wastewater treatment, and from metal electroplating to water treatment, they are ubiquitous and provide crucial support for the development of numerous electrochemical industries.
Its excellent corrosion resistance, high efficiency electrolytic performance, and long service life make it the preferred anode material for many electrochemical processes. However, to fully utilize the advantages of ruthenium iridium titanium anodes, proper selection and use are of crucial importance.
Core composition and advantages
Unique ingredients
An alloy material composed of three metals: ruthenium (Ru), iridium (Ir), and titanium (Ti), with each component playing an irreplaceable role.
High performance
Low resistivity, good electrical conductivity, and excellent corrosion resistance ensure the efficient progress of the electrolytic reaction.
Widely applied
Numerous electrochemical fields such as the chlor-alkali industry, electroplating industry, sewage treatment, and salt chlorine generators.
How to choose a ruthenium iridium titanium anode
The ruthenium-iridium-titanium anode, as its name implies, is an alloy material composed of three metals: ruthenium (Ru), iridium (Ir), and titanium (Ti). In this combination, each metal plays an irreplaceable role:
- Ruthenium: Can significantly enhance the electrocatalytic activity of materials, allowing electrochemical reactions to proceed more efficiently.
- Iridium: enhances the stability and corrosion resistance of materials, enabling them to remain stable in complex chemical environments.
- Titanium: As the matrix, it provides the necessary strength and toughness for the entire alloy.
excellent performance
- Low resistivity, with good electrical conductivity, enabling efficient current transmission.
- Excellent corrosion resistance, with the best stability in a neutral to weakly acidic environment.
- Longer service life, reducing the replacement frequency and maintenance costs.
- Good electrocatalytic performance, reducing overpotential and accelerating the reaction rate.
Main application fields
- Chlor – alkali industry: electrolytic production of chlorine, sodium hydroxide and hydrogen.
- Electroplating industry: Make the coating more uniform and dense, and improve the quality.
- Sewage treatment: Achieving sewage purification and recycling.
Salt chlorine generator: Efficiently generates chlorine gas with bactericidal and disinfectant effects.
Selection Guide: Find the Most Suitable One
1.Determine the direction based on the application scenario
- Chlor – alkali industry
The anode is required to have excellent chlorine evolution performance and high current efficiency to ensure the high efficiency and stability of production. At the same time, since it will come into contact with high – concentration chloride ions and an alkaline environment during the production process, the corrosion resistance of the anode is also of great importance. - The field of metal electroplating
Whether it is decorative electroplating or functional electroplating, the uniformity, compactness, and glossiness of the coating are pursued. At this time, the ruthenium-iridium-titanium anode needs to have the ability to accurately distribute the current to ensure a uniform electroplating effect on plated parts of different shapes and sizes. - Wastewater treatment
The processing objects are complex and diverse. Ruthenium-iridium-titanium anodes need to have strong electrocatalytic oxidation ability to generate reactive species with strong oxidizing power, which can efficiently decompose organic pollutants. At the same time, they should also have good stability to cope with the complex chemical substances and variable water quality conditions in sewage. - Salt chlorine generator and sodium hypochlorite generator
In fields such as swimming pool water treatment and small – scale aquaculture, salt – chlorine generators produce chlorine or hypochlorous acid with bactericidal and disinfecting effects by electrolyzing saline solution. The ruthenium – iridium – titanium anode directly affects the chlorine – production efficiency, stability, and service life of the equipment. - Seawater electrolysis
It is mainly used for seawater chlorination, seawater desalination, etc. Seawater is rich in a large amount of salt and various minerals and has strong corrosiveness. Therefore, the anode is required to have extremely high seawater corrosion resistance, and at the same time, it needs to have high electrolysis efficiency.
In the actual selection process, these performance indicators often need to be weighed and balanced. It is necessary to determine the priority of each performance indicator according to specific application requirements and find the best balance point.
2.Consider performance indicators when making decisions
Current efficiency
It directly affects the energy consumption and production efficiency of the electrolysis process. A high current efficiency means that more target products can be produced with the same amount of electricity input, reducing energy waste.
Overpotential
The difference between the actual potential and the theoretical potential of the anode in an electrochemical reaction reflects the ease of the electrode reaction. A low overpotential can reduce the energy consumption in the electrolysis process.
Corrosion resistance
The ruthenium iridium titanium anode ensures stable operation in various complex chemical environments. Anodes with different coating formulations and thicknesses can be selected according to the specific operating environment.
Corrosion resistance
The ruthenium iridium titanium anode ensures stable operation in various complex chemical environments. Anodes with different coating formulations and thicknesses can be selected according to the specific operating environment.
3.Make wise choices by referring to cost factors
Cost is an important factor that enterprises cannot ignore when choosing ruthenium-iridium-titanium anodes. The cost of ruthenium-iridium-titanium anodes mainly consists of raw material costs, manufacturing costs, and service life costs.
Cost Composition
- Raw material cost
Accounting for a relatively large proportion, ruthenium and iridium, as precious metals, have relatively high prices. Their content and proportion directly affect the cost of the anode. - manufacturing cost
Including the costs of coating preparation, processing and molding, advanced manufacturing processes and strict quality control will increase the manufacturing costs. - Life Cycle Cost
Replacement frequency and maintenance costs during long – term use. Although high – performance anodes have a high initial cost, they have a long lifespan and are more cost – effective in the long run.
Under the premise of meeting performance requirements, we can adopt some strategies to balance cost and performance. Firstly, according to the actual application scenario, the content ratio of ruthenium and iridium can be reasonably selected. Both ruthenium and iridium are precious metals, which makes the cost of ruthenium-iridium-titanium anodes relatively high. When selecting the model, we cannot blindly pursue low prices while ignoring the quality and service life of the anode. Although high-quality ruthenium-iridium-titanium anodes have a relatively high initial cost, they have a long service life, can reduce the replacement frequency and maintenance costs, and are more economical in the long run. Secondly, choosing the appropriate manufacturing process and supplier is also very important. Different manufacturing processes may lead to differences in product quality and cost. By comparing and screening, and choosing a supplier (QI XIN TITANIUM) with a long history of operation, the replacement frequency and maintenance costs of the anode can be effectively reduced, and it is more cost-effective in the long run.
Precautions for use: Extending lifespan and improving efficiency
The correct selection is the foundation for giving play to the performance of ruthenium-iridium-titanium anodes, while reasonable use and maintenance are the keys to ensuring their long-term stable operation, extending their service life, and improving work efficiency.
- Before installing the ruthenium-iridium-titanium anode, meticulous inspection work is essential. First of all, conduct a comprehensive inspection of the appearance of the anode to check whether there are defects such as scratches, abrasions, and coating peeling on the surface. Even the slightest scratch may become the starting point of corrosion. Over time, it will gradually expand and affect the performance and service life of the anode. At the same time, ensure that it precisely matches the design dimensions of the electrolytic cell. Inconsistent dimensions may lead to insecure installation, causing shaking during the electrolysis process, thus affecting the uniformity of current distribution and reducing the electrolysis efficiency.
The installation process must be carried out strictly in accordance with the operating procedures. First, place the anode steadily in the preset position inside the electrolytic cell, ensuring that it is in the central position to avoid uneven current distribution due to position deviation. Then, adopt an appropriate fixing method, such as using insulating brackets, screws, etc., to firmly fix the anode to prevent the anode from moving during the electrolysis process due to factors such as the flow of the electrolyte and the generation of gas. During the fixing process, pay attention to applying moderate force to avoid damaging the anode by over-tightening the screws.
In addition, in order to prevent galvanic corrosion, it is necessary to avoid direct contact between the anode and other metals. Insulating materials such as plastic gaskets and rubber pads can be added between the anode and other metal components. Galvanic corrosion is a corrosion phenomenon caused by the formation of a potential difference between different metals in an electrolyte solution. Once galvanic corrosion occurs, the corrosion rate of the anode will be greatly accelerated, seriously affecting its service life. In practical applications, even the smallest metal contact point may trigger galvanic corrosion, so this issue must be highly emphasized. - During the electrolysis process, it is crucial to reasonably control various operating parameters, as these parameters directly affect the performance and service life of the anode. Current density is a key parameter, which is closely related to the chlorine evolution reaction of the anode. An excessively high current density will make the reaction on the anode surface too intense, resulting in a rapid increase in the anode temperature, accelerating the loss of the coating, and shortening the service life of the anode. On the other hand, an excessively low current density will reduce the electrolysis efficiency and increase the production cost. Different application scenarios have different requirements for current density. In the chlor – alkali industry, the current density is generally controlled between 2 – 5 kA/m². This can not only ensure a high electrolysis efficiency but also enable the anode to maintain stable performance.
The control of voltage cannot be ignored either. If the voltage is too high, it will not only increase energy consumption but may also cause the anode to passivate. Anode passivation refers to the formation of a passivation film with a very high resistance on the anode surface, which causes the electrode potential of the anode to rise sharply, preventing the normal passage of current and thus losing its electrocatalytic activity. Once passivation occurs, the performance of the anode will decline significantly or even completely fail. In order to avoid anode passivation, it is necessary to reasonably adjust the voltage according to the specific electrolysis system and process requirements to ensure it is within an appropriate range.
The influence of temperature on anode performance is also very significant. Generally speaking, as the temperature increases, the conductivity of the electrolyte will increase, and the rate of electrochemical reactions will accelerate, which is conducive to improving the electrolysis efficiency. However, if the temperature is too high, it will exacerbate the corrosion of the anode and may also cause the evaporation of the electrolyte and changes in its composition. For most application scenarios of ruthenium – iridium – titanium anodes, the appropriate temperature range is usually between 30 – 60°C. In actual operation, temperature – control devices such as thermometers and cooling systems can be installed to monitor and adjust the temperature of the electrolyte in real – time to ensure it remains stable within the appropriate range.
The concentration and pH value of the electrolyte also have an important impact on the anode. Different electrolyte concentrations will change the reaction kinetics on the anode surface, thus affecting the performance of the anode. If the concentration of certain ions in the electrolyte is too high, it may lead to the formation of precipitates on the anode surface, hindering the progress of the electrochemical reaction. Changes in the pH value will affect the corrosion potential and reaction activity of the anode. An overly acidic or alkaline environment may accelerate the corrosion of the anode. In the electroplating industry, it is necessary to accurately control the concentration and pH value of the electrolyte according to different electroplating processes and bath compositions to ensure the normal operation of the anode and the quality of electroplating. - Daily maintenance is an important part of ensuring the continuous and stable operation of the ruthenium-iridium-titanium anode. The surface condition of the anode should be carefully inspected regularly. By observing whether there are abnormal phenomena such as deposits, discoloration, and coating peeling on the anode surface, check the integrity of the coating and changes in the surface structure.
Once deposits and impurities are found on the anode surface, they should be removed in a timely manner. For some loose deposits, they can be cleaned by low-pressure water flushing. Pay attention that the water pressure should not be too high to avoid damaging the anode coating. For more stubborn deposits, appropriate chemical reagents can be selected for cleaning according to the composition of the deposits. If the deposits are mainly metal hydroxides, they can be soaked and cleaned with a dilute acid solution, but the cleaning time and acid concentration should be strictly controlled to avoid corroding the anode. After cleaning, the anode should be rinsed with a large amount of clean water to ensure that there is no residual chemical reagent on the surface.
When the anode is damaged, such as large-area coating peeling, substrate corrosion, etc., it must be replaced in a timely manner. Continuing to use the damaged anode will not only affect the electrolysis efficiency and product quality, but may also cause malfunctions in the entire electrolysis system. When replacing the anode, choose a product with the same specifications and performance as the original anode, and install it according to the correct installation steps.
In addition, maintaining the electrolytic cell and related equipment also has an important impact on the anode performance. Regularly check the tightness of the electrolytic cell to prevent electrolyte leakage; inspect and tighten the electrode connection parts to ensure good contact and reduce resistance loss; promptly clean the debris and deposits in the electrolytic cell to keep the electrolyte clean. Only when the entire electrolysis system is in good operating condition can the ruthenium-iridium-titanium anode fully exert its performance advantages and achieve efficient and stable operation.
Pay special attention
Galvanic corrosion is a corrosion phenomenon triggered by the formation of a potential difference between different metals in an electrolyte solution. Once galvanic corrosion occurs, the corrosion rate of the anode will be greatly accelerated, seriously affecting its service life. In practical applications, even the smallest metal contact points can potentially trigger galvanic corrosion, so this issue must be taken highly seriously.

Selection Guide: Find the Most Suitable One
Anodic passivation
Anodic passivation is a phenomenon that may occur during the use of ruthenium-iridium-titanium anodes. When the voltage rises to a certain level and there is actually no current passing through, the anode loses its function, and this phenomenon is called anodic passivation. The occurrence of anodic passivation will seriously affect the electrolysis efficiency and the normal progress of production.
Reasons for Anodic Passivation
- Coating peeling:If the coating is not firmly bonded to the substrate and detaches from the titanium substrate, when the detachment reaches a certain extent, the anode will lose its function. The forms of detachment include pulverized detachment, belly – shaped delamination, and cracking – type detachment, etc.
- Dissolution of RuO₂:RuO₂ is an important component in the coating of ruthenium-iridium-titanium anodes. Its dissolution reduces the evolution of oxygen, thereby slowing down the formation of the oxide film. If RuO₂ dissolves excessively, it will destroy the electrocatalytic activity of the anode, leading to anode passivation.
- oxide saturation:When the oxygen vacancies in the active coating are filled with oxygen, the overpotential rises rapidly, leading to passivation. The non-stoichiometric oxides are the true activation centers for chlorine discharge. The more such oxides there are, the more active centers there will be.
- There are cracks in the coating:The electrolyte invades through the cracks in the coating, the titanium substrate is gradually oxidized, the interface between the ruthenium-iridium-titanium active coating and the substrate is corroded, causing the coating to peel off. This leads to an increase in the anode potential and ultimately results in anode passivation.
- Optimize coating preparation process: By improving the coating preparation process, such as adopting appropriate coating methods and optimizing heat treatment conditions, to enhance the bonding strength between the coating and the substrate, reducing the possibility of coating peeling.
- Control operating parameters: Reasonably control operating parameters such as current density, voltage, and temperature to avoid anode passivation caused by improper parameters. Develop scientific operating specifications according to different electrolysis systems and process requirements, and implement them strictly.
- Regular maintenance and inspection: Establish a regular maintenance and inspection system to promptly detect issues such as sediment on the anode surface and coating cracks, and take corresponding measures to address them. Regularly clean and inspect the anode to ensure its surface is clean and the coating is intact.
Coating damage
The coating of the ruthenium-iridium-titanium anode may be damaged during use, such as peeling off, cracking, etc., which will affect the performance and service life of the anode.
Reasons for coating damage
- Mechanical damage:During the installation, disassembly or use process, the anode may be subject to mechanical impacts, friction, etc., resulting in damage to the coating. When handling the anode, if the operation is improper, the anode may collide with other objects, causing the coating to peel off or crack.
- chemical corrosion:Various chemical substances in the electrolyte may corrode the anode coating, especially in strongly acidic or alkaline environments, where the corrosion effect is more pronounced. Some electrolytes contain a high concentration of chloride ions, which will accelerate the corrosion of the coating.
- Thermal stress:During the electrolysis process, heat is generated at the anode. The change in temperature can cause thermal stress between the coating and the substrate. When the thermal stress exceeds a certain limit, it will lead to coating cracking or peeling.
Ruthenium Iridium Titanium anode
The ruthenium iridium titanium anode exhibits excellent corrosion resistance. It has the best stability in a neutral to weakly acidic environment or even in a saline seawater environment, and can operate stably for a long time without being rapidly consumed due to corrosion. This characteristic makes it stand out in applications that require long-term contact with corrosive media, greatly reducing the equipment maintenance cost and extending the service life of the equipment. Take the chlor-alkali industry as an example. Traditional anode materials are easily corroded during the electrolysis process and need to be replaced frequently. However, the emergence of the ruthenium-iridium-titanium anode has effectively solved this problem. Its service life has been significantly extended, from the original several months to several years, effectively reducing the equipment maintenance cost and the risk of production interruption.
The ruthenium iridium titanium anode has excellent electrocatalytic properties. It can reduce the overpotential, accelerate the rate of electrochemical reactions, and improve the electrolysis efficiency. In sewage treatment, through electrocatalytic oxidation technology, it can generate highly oxidizing active species such as hydroxyl radicals, efficiently decompose various organic pollutants in sewage, such as phenols, anilines, and pesticide residues, and convert them into harmless substances such as carbon dioxide and water. At the same time, it can also oxidize and precipitate or reduce and remove heavy metal ions in water, thus significantly reducing the toxicity of sewage, improving the sewage treatment efficiency, and ensuring that the effluent water quality meets the standards.
Precisely because of these outstanding properties, the ruthenium-iridium-titanium anode has been widely used in many fields. In the chlor-alkali industry, it is the core material for the electrolytic production of chlorine, sodium hydroxide, and hydrogen. In the electroplating industry, it can make the coating more uniform and dense, improving the quality and aesthetics of metal products. In the field of sewage treatment, it is the key to achieving sewage purification and recycling. In the salt-chlorine generator industry, the chlorine evolution performance of the anode is a core indicator. An anode with a low chlorine evolution overpotential can generate more chlorine under the same current conditions, improving the chlorine production efficiency. It can be said that the ruthenium-iridium-titanium anode has become an indispensable and important material in the modern electrochemical field.