Titanium anode system for chlorine production by seawater electrolysis
Titanium anode for chlorine production by seawater electrolysis generate sodium hypochlorite solution through the electrolysis of seawater containing a certain concentration of chloride ions. Adding sodium hypochlorite to circulating water can inhibit the growth and reproduction of microorganisms, thereby protecting the circulating water system.
The electrolytic chlorine generation system is used for controlling alien biological contamination in power plants, cooling towers, liquefied natural gas (LNG) terminals, desalination facilities, coastal equipment that uses seawater as cooling water, and other processes.
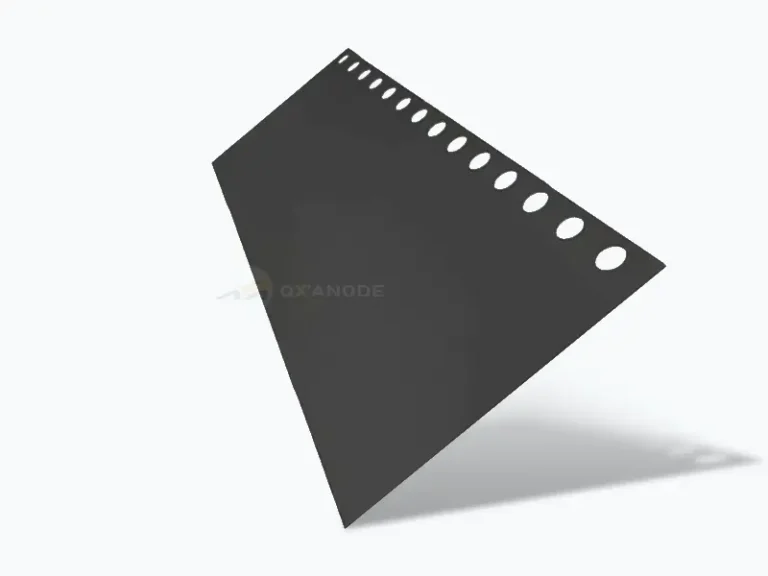

Titanium anode for chlorine production by seawater electrolysis
Substrate | Gr1/Gr2 |
Types of coatings | Ruthenium iridium coated titanium anode |
Electrolysis temperature | 20℃~40℃ |
Electrode cleaning solution | Dilute hydrochloric acid |
Electrode cleaning cycle | 1-2 months |
Seawater flow rate | 343/h 273/h |
As the core component of this technology, the performance optimization of titanium anodes is directly related to the efficiency and stability of the system. During the seawater electrolysis process, the strong corrosiveness of chloride ions (Cl⁻) and bromide ions (Br⁻) poses severe challenges to anode materials. Traditional metal anodes struggle to meet the requirements of long-term operation due to issues such as easy corrosion and short service life. Titanium anodes, with their excellent corrosion resistance, mechanical strength, and electrochemical stability, have become the mainstream choice in current research and applications.
The performance optimization of titanium anode materials is a core link in improving their stability and efficiency in the process of electrolyzing seawater to produce chlorine. Adjustments in the preparation process directly affect the coating structure, element distribution, and surface properties, which in turn significantly influence the kinetic process of the chlorine evolution reaction and corrosion resistance. By introducing intermediate layer modification technology, the bonding strength between the titanium substrate and the active coating can be effectively enhanced, preventing structural spalling caused by high-temperature sintering or long-term electrolysis. Experiments show that the design of a multi-layer gradient coating can significantly improve the mechanical strength and conductivity of the electrode. For example, pre-depositing a transition metal oxide intermediate layer on the surface of the titanium substrate can form a more uniform electron transport channel, thereby reducing interface resistance and improving current efficiency.


Electrolytic cell cover: The cell cover is made of transparent organic glass. During operation, operators can directly observe the reaction inside the cell through the cover, which helps to more accurately grasp the timing for electrolytic cell maintenance and pickling.
Electrolyzer shell: The shell is made of polyvinyl chloride material, which is highly resistant to sodium hypochlorite corrosion. This structure has high safety and stability, and the specially designed O-ring seal eliminates the problem of liquid leakage.
Anode: The anode uses a dimensionally stable anode (DSA) with a titanium substrate coated with precious metal oxides. This anode exhibits excellent electrochemical performance within the temperature range of 0~45℃ and has a long service life. The anode is in the shape of a plate grid, and the anode and cathode are separated by a PVDF spacer with a distance of 2.5mm maintained between them.
The anode in the electrolytic cell is of the monopolar type. The electrolytic cell is placed vertically, and seawater flows through it at a high speed from bottom to top in a single pass. The hydrogen gas generated by electrolysis can be smoothly discharged along with the water flow without accumulating in the cell. The high flow rate slows down the accumulation of calcium and magnesium precipitates on the cathode, thereby extending the pickling cycle.
Conductivity: The conductive connecting parts of the cathode or anode in the electrolytic cell are titanium-copper composite rods. The part of the composite rod inside the cell is titanium, and the part outside the cell is copper. This not only avoids corrosion but also ensures good conductivity. The electrical connection between cells uses copper plates, making disassembly very simple and quick.
Application of Titanium Anodes for Chlorine Production by Seawater Electrolysis
Biofouling Prevention in Coastal Power Plant / Nuclear Power Cooling Systems
Coastal power plants and nuclear power plants use seawater as a cooling medium (with a circulating water volume of up to 10⁴–10⁵ m³/h). Seawater is rich in microorganisms such as shellfish, algae, and bacteria, which tend to attach and grow on the inner walls of condensers and pipelines. This leads to a decrease in heat exchange efficiency (by up to 30%), pipeline blockages, and even equipment corrosion and leakage, seriously affecting the safe operation of the units.
- Ruthenium-based (Ru-Ir-Sn) titanium anodes (balancing activity and cost) are used, combined with diaphragmless electrolytic cells. Through the electrolysis of seawater, effective chlorine such as Cl₂ and NaClO (with a concentration of 0.5–1.0mg/L) is generated and injected into the cooling system to kill microorganisms.
- Electrolysis parameters: current density 0.8–1.5kA/m², cell voltage 2.8–3.5V, effective chlorine production rate ≥8g/(h・m²), ensuring anti-fouling effect while controlling energy consumption.
- The anode adopts a plate/mesh structure (to increase the specific surface area) and is equipped with an online residual chlorine monitor to automatically adjust the electrolysis current and maintain a stable residual chlorine concentration.
Ballast water / cooling system antifouling and disinfection
The cross-sea transportation of ship ballast water is prone to introducing alien invasive species, causing ecological damage; ship cooling pipelines also face the problem of microbial adhesion, which affects navigation safety. The International Maritime Organization (IMO) requires ballast water to meet the “D-2 Standard” (viable count ≤ 10 CFU/mL).
Regular acid cleaning
When electrolyzing seawater, in addition to producing sodium hypochlorite and hydrogen, calcium and magnesium precipitates are inevitably generated, which accumulate on the cathode of the electrolytic cell. This leads to an increase in the cell voltage of the electrolytic cell, a decrease in current efficiency, and an increase in power consumption. Therefore, it is necessary to regularly pickle the electrolytic cell to remove the precipitates on the cathode surface. The pickling cycle of the electrolytic cell is generally 30 days. During pickling, first, seawater or tap water is injected into the pickling tank to a certain height, and then the seawater in the tank is made to flow through the calculated and checked small holes in the proportioner (injector) at a high speed by a centrifugal plastic pump, thereby drawing hydrochloric acid into the pickling tank. Seawater is injected again to adjust the concentration of the hydrochloric acid solution to 10%. Then, the dilute hydrochloric acid is circulated between the pickling tank and the electrolytic cell group. After pickling, the pickling pump draws the waste acid accumulated in the electrolytic cell group back into the pickling tank, which is finally neutralized and discharged, and the pickling is completed.
- Ballast water disinfection: A modular titanium anode electrolysis device is used, with a treatment flow rate adapted to the ship’s ballast capacity (50–500 m³/h). It generates 5–10 mg/L of available chlorine, with a contact time of ≥30 minutes, and is combined with activated carbon filtration to remove residual chlorine.
- Anti-fouling for cooling systems: Ruthenium-iridium-titanium anodes are selected to adapt to the special working conditions of ships. The anodes adopt a corrosion-resistant fixed structure to avoid coating scratches.
- The anode must withstand fluctuations in seawater salinity, temperature changes (5–30℃), as well as be resistant to impact and vibration.

Titanium anode for chlorine production by seawater electrolysis improve the stability and electrolysis efficiency of the system during the process of producing chlorine gas through seawater electrolysis. Appropriate adjustments to the preparation process of titanium anodes will directly affect their coating structure, element distribution, and surface properties, thereby significantly influencing the reaction rate of chlorine gas generation and the corrosion resistance of the material. By adopting various modification technologies, the bonding strength between the titanium substrate and the active coating can be effectively enhanced, thus avoiding structural spalling caused by high-temperature sintering or long-term electrolysis. The use of a multi-layer gradient coating design can also significantly improve the mechanical strength and electrical conductivity of the electrode, make the electron transport channels more uniform, thereby reducing interface resistance and increasing current utilization efficiency.
If you encounter any problems in the practical application of titanium anode for chlorine production by seawater electrolysis, or have unique insights into their future development—whether it’s technical difficulties or discussions on industry trends—please feel free to leave a message below. We will communicate with you here and jointly explore the unlimited possibilities of titanium anodes.



