Lead dioxide titanium anode
The lead dioxide titanium anode has extremely high electrochemical activity, which can effectively promote the progress of chemical reactions during the electrolysis process. Secondly, this anode material has excellent stability and can work stably for a long time in a harsh electrochemical environment, thus ensuring the continuity and stability of the electrolysis process. In addition, the lead dioxide titanium anode also has good electrical conductivity and corrosion resistance, further enhancing its reliability in practical applications.
In the field of water treatment, it can be used as the anode material in the electrochemical oxidation method to effectively remove organic pollutants and heavy metal ions in water and improve water quality. During the electrochemical oxidation process, the lead dioxide-titanium anode can efficiently generate reactive substances such as hydroxyl radicals with strong oxidizing properties, thus achieving rapid degradation of pollutants. At the same time, its high stability and corrosion resistance also ensure the continuity and stability of the water treatment process.
In addition to the water treatment field, the lead dioxide titanium anode also exhibits remarkable effects in the removal of anionic pollutants. Through electrochemical methods, this anode can effectively remove anionic pollutants such as nitrates and phosphates from wastewater, thereby further purifying the water quality and protecting the environment.
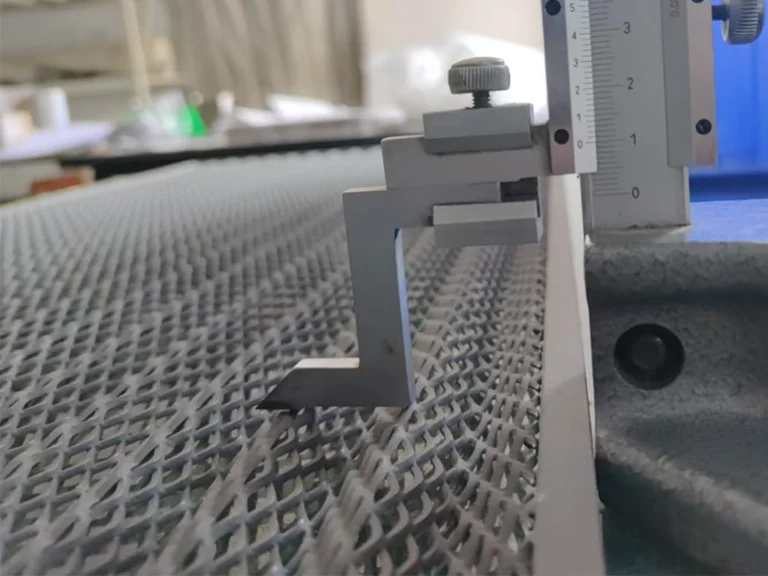
Basic Concept of Lead titanium dioxide anodes
Lead dioxide titanium anode, as β composite anode material, is prepared by electroplating lead dioxide on the surface of a titanium mesh. This material combines the excellent properties of titanium dioxide and lead dioxide, exhibiting high electrochemical activity, outstanding corrosion resistance, and stability, enabling it to perform excellently in various electrochemical environments. Its unique composition and structure endow the lead dioxide titanium anode with remarkable electrochemical characteristics. Therefore, in the field of electrochemistry, it has a broad range of applications.
In terms of electrochemical performance, the lead dioxide-titanium anode exhibits extremely high electrochemical activity and stability. Its high activity enables the anode to provide sufficient reaction sites in electrochemical reactions, thereby accelerating the progress of the reactions. Meanwhile, its excellent stability ensures that the anode can maintain stable performance during long-term use, extending the service life of the material. These performance characteristics give the lead dioxide-titanium anode significant advantages and application potential in the fields of water treatment, electrochemical oxidation, hydrometallurgy, etc.
Application fields of Lead dioxide titanium anodes
Lead dioxide-titanium anode in the field of water treatment. With the rapid development of industrialization and urbanization, water pollution has become increasingly serious, making effective water treatment technologies particularly important. The lead dioxide-titanium anode, with its excellent oxidation performance, can efficiently remove organic matter, heavy metal ions and other pollutants from wastewater, thus achieving the purification and resource utilization of wastewater. In terms of water supply disinfection, this anode material also performs excellently, which can effectively kill pathogenic microorganisms in water and ensure water quality safety.
In the treatment of phenolic wastewater decolorization, oilfield wastewater, printing and dyeing wastewater, and ammonia-nitrogen wastewater, lead dioxide-titanium anodes have demonstrated remarkable treatment effects. Their high-efficiency electrocatalytic oxidation capability can rapidly decompose organic substances in these wastewaters, thus achieving harmless treatment and up-to-standard discharge of the wastewater.
In chemical engineering fields such as smelting and persulfate production, lead dioxide-titanium anodes also play an important role. Their high electrochemical activity and strong oxidation capability enable these chemical reactions to proceed more efficiently and safely. Meanwhile, the corrosion resistance and stability of this anode material greatly reduce equipment maintenance costs and production risks.
Examples of the Application of Lead dioxide
- In a wastewater treatment project of a certain chemical industrial park, lead-titanium dioxide anodes were applied in the electrochemical oxidation method to treat refractory organic compounds. In this project, the wastewater contained a large amount of organic compounds that were difficult to be biodegraded. The traditional biological treatment methods were ineffective. By introducing lead-titanium dioxide anodes for electrochemical oxidation treatment, not only did it effectively remove the organic compounds from the wastewater, but also significantly improved the biodegradability of the wastewater, creating favorable conditions for subsequent biological treatment.
- In the wastewater treatment of another dyeing enterprise, lead-titanium dioxide anodes were used to remove dyes and auxiliaries from the wastewater. Dyeing wastewater usually contains a large amount of difficult-to-degrade dyes and chemical auxiliaries, which have a significant impact on the environment. By using lead-titanium dioxide anodes for electrolytic treatment, the dyes and chemical auxiliaries in the wastewater were effectively removed, and at the same time, the color and COD of the wastewater were significantly reduced, meeting the environmental protection discharge standards.
- In the treatment of oilfield wastewater, the lead-titanium dioxide anode has also demonstrated excellent application effects. The wastewater from oilfields contains a large amount of oil and harmful substances, making the treatment process quite challenging. By using the lead-titanium dioxide anode for electrochemical treatment, not only are the oil and harmful substances in the wastewater effectively removed, but the biodegradability of the wastewater is also improved, providing favorable conditions for subsequent biochemical treatment.