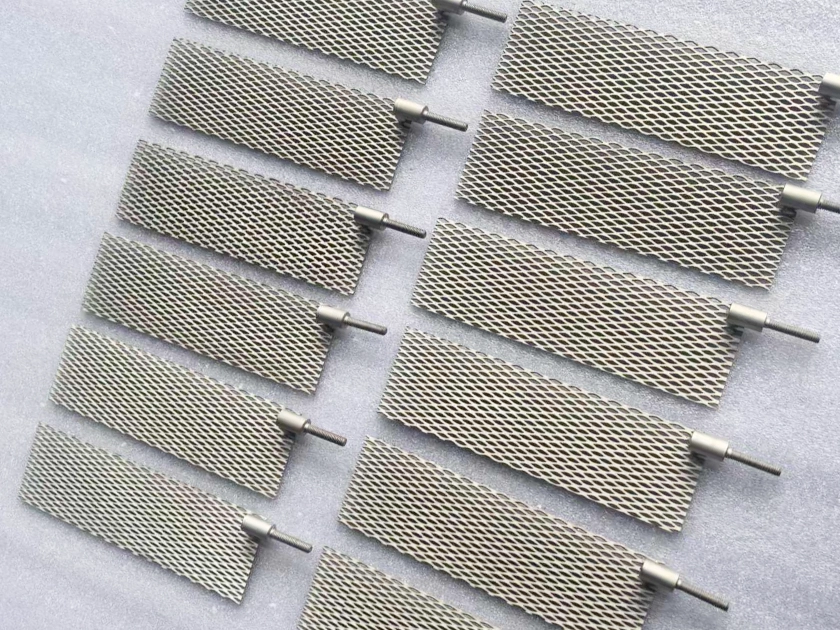
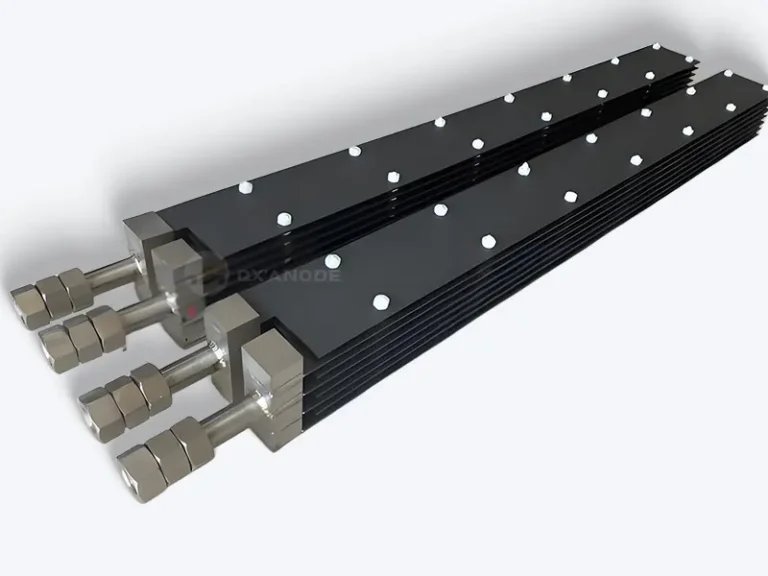
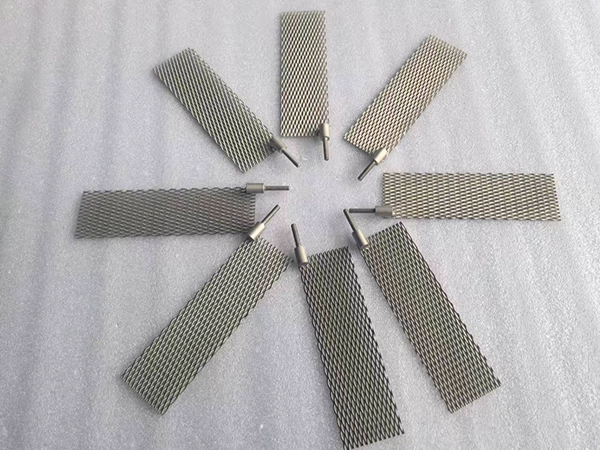
Platinum titanium anodes
Platinum titanium anodes, as a high-performance electrochemical material that combines the catalytic activity of platinum and the excellent mechanical properties of titanium, have shown broad application prospects in the field of electrochemistry. They perform well in organic synthesis, fuel cells, electrochemical sensors, and seawater desalination, among others, and have a promising future. In the future, with the strengthening of environmental regulations and continuous technological progress, the environmental performance and preparation efficiency of platinum titanium anodes will be further enhanced, and their applications in the field of electrochemistry will become more extensive. As a core electrode material in the electrochemical industry.
Platinum titanium anodes, with the corrosion resistance of the titanium substrate and the high electrocatalytic activity of the platinum coating, play a key role in the electrochemical industry. With the advancement of the global “carbon neutrality” goal, the demand for their application in efficient electrochemical processes has significantly increased.

Platinum titanium anodes are widely used in technologies such as fuel cells and electrolytic hydrogen production due to their highly efficient electrocatalytic activity. They can accelerate the rate of electrochemical reactions, thereby enhancing energy conversion efficiency. Additionally, in electrochemical synthesis, platinumn titanium anodes also demonstrate unique advantages.
They play a significant role in wastewater treatment as well. By leveraging their electrocatalytic oxidation performance, organic pollutants in wastewater can be effectively degraded, improving the biodegradability of the wastewater. Utilizing platinum titanium anodes for electrochemical treatment can significantly increase the removal rate of chemical oxygen demand (COD) in wastewater, providing a new and effective approach for wastewater treatment.

Platinum-titanium anodes, as a high-performance material in the field of electrochemistry, are prepared by coating a thin layer of platinum metal on the surface of a titanium substrate. This type of anode is typically composed of a titanium substrate and a layer of platinum metal attached to its surface through electroplating technology. During the preparation process, industrial pure titanium Gr1/Gr2 is commonly used as the titanium material, and the thickness of the platinum layer is usually between 0.5 and 5 microns. In some special working conditions, the thickness of the platinum layer may exceed 10 microns to meet more demanding application requirements.
In the electrochemical industry, platinum-plated titanium anodes are often used as electrode materials in electrolysis processes. Due to their excellent electrical conductivity and corrosion resistance, they can effectively promote the progress of electrolysis reactions and improve electrolysis efficiency. Meanwhile, the high catalytic activity of the platinum layer makes platinum-plated titanium anodes perform excellently in certain specific electrochemical reactions.
In the field of energy conversion and storage technologies, platinum-titanium anodes also demonstrate enormous application potential. For example, in fuel cells, platinum-titanium anodes can serve as catalysts for the oxygen reduction reaction, thereby enhancing the performance and efficiency of fuel cells. Meanwhile, due to their excellent corrosion resistance and stability, platinum-plated titanium anodes also play a significant role in renewable energy technologies such as hydrogen production via water electrolysis.
Platinum titanium anodes performance
In terms of electrochemical performance, polarization potential, corrosion potential, and corrosion current density are important indicators for measuring the performance of platinum-plated titanium anodes. The polarization potential reflects the activity of the anode in electrochemical reactions. A lower polarization potential means that the anode can start working at a lower potential, thereby improving energy utilization efficiency. The corrosion potential and corrosion current density are directly related to the corrosion resistance of the anode. A higher corrosion potential and a lower corrosion current density indicate that the anode has better stability in harsh environments. Through the comprehensive analysis of these indicators, we can comprehensively evaluate the performance of platinum-plated titanium anodes in electrochemical applications.
Platinum plated titanium anodes should possess high efficiency electrocatalytic activity. As the catalytic active center, the platinum layer plays a crucial role in electrochemical reactions. It needs to have high electrocatalytic activity to reduce the activation energy of the reaction, thereby improving the reaction rate and efficiency. Such high efficiency electrocatalytic activity is one of the key factors for the wide application of platinum-plated titanium anodes in the electrochemical field. For example, in the process of electrochemical organic synthesis, the high catalytic activity of the platinum layer can significantly increase the yield and reduce the production cost.
Platinum plated titanium anodes need to have excellent corrosion resistance. In harsh electrochemical environments, anode materials are often prone to corrosion, which affects their performance and service life. Therefore, both the titanium substrate and the platinum layer of platinum-plated titanium anodes should possess excellent corrosion resistance to ensure their stability and reliability during long term operation. This corrosion resistance gives platinum-plated titanium anodes significant advantages in hydrogen production by electrolysis and carbon neutrality.
Good electrical conductivity is also one of the indispensable properties of platinum-plated titanium anodes. In electrochemical reactions, the anode material needs to have high electrical conductivity to ensure that the current can pass smoothly, thereby maintaining the smooth progress of the reaction. Platinum plated titanium anodes achieve high electrical conductivity through optimizing the preparation process and material composition, providing a strong guarantee for the efficient progress of electrochemical reactions.
Platinum plated titanium anodes should also possess stable mechanical properties. During electrochemical reactions, anode materials often need to withstand high current densities and mechanical stresses. Therefore, platinum-plated titanium anodes are required to have stable mechanical properties to ensure their integrity and stability under high-load conditions. Such stable mechanical properties endow platinum-plated titanium anodes with broad application prospects in industrial fields such as electroplating and electrolysis.
The requirements for platinum plated titanium anodes, including high-efficiency electrocatalytic activity, excellent corrosion resistance, good electrical conductivity, and stable mechanical properties, collectively ensure their outstanding performance and wide application in the electrochemical field.